High-performance liquid chromatography
Polymerase Chain Reaction (PCR)
Amplification means making multiple identical copies (replicates) of a DNA sequence. This can be carried out by various methods, that include in vivo amplification including cell cloning where host cells (manipulated using a vector to contain a DNA insert of interest) are allowed to divide and, as they do so, the insert is replicated.
Historical background…
- Method first proposed by H. G. Khorana & colleagues in 1970’s.
- 15 years later the idea was independently conceived by Karry Mullis in 1983.
- Used the Klenow fragment of E. coli DNA polymerase to describe the in-vitro amplification of genes.
- Saiki et al in 1988 used the thermostable DNA polymerase from Thermus aquaticus and greatly increased the efficiency of PCR.
- In 1989, Science magazine selected PCR as the major scientific development and Taq DNA polymerase as the molecule of the year.
- Karry Mullis was awarded the Noble price for chemistry in 1993.
- Template DNA
- A thermostable DNA polymerase
- A pair of synthetic oligonucleotide primers.
- Divalent cations (Mg 2+ )
- dNTPs
- Buffer to maintain pH ( Tris-Cl pH 8.3 – 8.8)
- Monovalent cations
The PCR usually consists of a series of 30 to 35 cycles. Most commonly, PCR is carried out in three steps, often preceded by one temperature hold at the start and followed by one hold at the end. A typical PCR cycle has following steps
Cell based assay for Drug discovery.
Radioimmunoassay (RIA)
MTT Assay for Cell Viability
MTT and related tetrazolium salts
MTT Assays significance
Selection, Gene amplification and cell line screening
Protein Glycosylation
The Polymerase Chain Reaction (PCR)
Since this technique involves amplification of DNA, the most obvious application of the method is in the detection of minuscule amounts of specific DNAs. This important in the detection of low level bacterial infections or rapid changes in transcription at the single cell level, as well as the detection of a specific individual's DNA in forensic science. It can also be used in DNA sequencing, screening for genetic disorders, site specific mutation of DNA, or cloning or subcloning of cDNAs.
The Reaction
PCR, like DNA sequencing, is based on the DNA polymerization reaction. A primer and dNTPs are added along with a DNA template and the DNA polymerase (in this case, Taq). The main difference with PCR is that, in addition to using a primer that sits on the 5' end of the gene and makes a new strand in that direction, a primer is made to the opposite strand to go in the other direction. The original template is melted at 94 Degree C, the primers anneal at 45-55 Degree C and the polymerase makes two new strands at 72 Degree C, doubling the amount of DNA present. This provides 2 new templates for the next cycle. The DNA is again melted, primers anneal, and the Taq makes 4 new strands:
Notice:
*Every cycle results in a doubling of the number of strands DNA present.
*After the first few cycles, most of the product DNA strands made are the same length as the distance between the primers.
The result is a dramatic amplification of a the DNA that exists between the primers. These cycles are repeated 20 to 40 times, each cycle providing 2 new templates for the next cycle. The amount of amplification is 2 raised to the n power; n represents the number of cycles that are performed. After 20 cycles, this would give approximately 1 million fold amplification. After 40 cycles the amplification would be 1 X 10^12. The reaction is performed in a thermocycler, which is programmable heating block that will cycle between melting, annealing and polymerization temperatures.
Limitations/Difficulties
While a very powerful technique, PCR can also be very tricky. The polymerase reaction is very sensitive to the levels of divalent cations (especially Mg2+) and nucleotides, and the conditions for each particular application must be worked out.
Primer design is extremely important for effective amplification. The primers for the reaction must be very specific for the template to be amplified. Cross reactivity with non-target DNA sequences results in non-specific amplification of DNA.
Also, the primers must not be capable of annealing to themselves or each other, as this will result in the very efficient amplification of short nonsense DNAs.
The reaction is limited in the size of the DNAs to be amplified (i.e., the distance apart that the primers can be placed). The most efficient amplification is in the 300 - 1000 bp range, however amplification of products up to 4 Kb has been reported. Also, Taq polymerase has been reported to make frequent mismatch mistakes when incorperating new bases into a strand.The most important consideration in PCR is contamination. If the sample that is being tested has even the smallest contamination with DNA from the target, the reaction could amplify this DNA and report a falsely positive identification. For example, if a technician in a crime lab set up a test reaction (with blood from the crime scene) after setting up a positive control reaction (with blood from the suspect) cross contamination between the samples could result in an erroneous incrimination, even if the technician changed pipette tips between samples. A few blood cells could volitilize in the pipette, stick to the plastic of the pipette, and then get ejected into the test sample. The powerful amplification of PCR may be able to detect this cross contamination of samples. Modern labs take account of this fact and devote tremendous effort to avoiding this problem.
Procedure:
Primers
As stated above, the selection of primers is very important to the efficiency of the reaction. Usually the primers are custom synthesized based on the sequence of the DNA that is being amplified. In your reactions, two primers would have to be made for each of the inserts and the primers that you use would be based on which insert you have in your plasmid. However, since all of the inserts are in the pBluescript plasmid, we can take advantage of the vector sequences that are common to all of the plasmids. For this reason you will all be using the same primers; one primer from the vector sequences at the 5' end of your insert and one from vector sequences at the 3' end of your insert. When the products are run on agarose gel they should each be the size of insert that you predicted from your restriction mapping.
Dilutions
This lab involves doing a serial dilution (see the lambda phage lab) of your isolated plasmid (from lab # 4), setting up 2 PCR reactions with this diluted template, running the PCR in the thermocycler, and then sizing the resultant fragments by agarose gel electrophoresis. This whole procedure should take about 6 hrs., so it will be done over two weeks. The first week, you will do the serial dilutions, set up the reactions and put the reactions in the thermocycler. The next week you will run the reactions out on an agarose gel.
Note: If your insert is greater than 2.0 Kb, tell me and I will give you a different plasmid because this is too large for efficient amplification.
Classification of Bacteria
OBLIGATE ANAEROBES: Do not required O2. E.g. Clostridium species.
FACULTATIVE AEROBES: Mainly anaerobes
FACULTATIVE ANAEROBES: Mainly aerobes (E.coli, clostridium sporogenes)
MICROAEROPHILE: Require very low concentration of oxygen.
Anaerobes have absence of 3 enzymes which are present in aerobes.
(i) Superoxide demutase: Eliminate Superoxide radical.
(ii) Catalase: Breakdown of H2O2 H2O + O2
(iii) Peroxidase: H2O2 + Reduced substance H2O + Oxidised substance
So free radicals produced in anaerobes in O2 environment. So anaerobes dies.
* Optimum pH for bacterial growth is 6.5-7.5.
* Bacteria can not tolerate salt but fungi tolerates salt.
Psychrophiles: Below 25 degree C (vibrio species)
Mesophiles: 25-45 degree C
Thermophiles: Optimum 55-65 degree C. Thermophilus.
Acidophilic: Tolerate high acidic condition (Lactobacilli)
Basophilic: Tolerate Alkaline condition (Vibrio cholerae)
Terminology of Stereochemistry
Stereocenter: a carbon atom bearing 4 different atoms or group of atoms.
Chiral: any molecule that is nonsuperposable with its mirror image.
Enantiomers: stereoisomers that are non superposable mirror images.
Racemic mixture: a 1:1 (equimolar) mixture of two enantiomers.
Optically Active: the ability of some compounds to rotate plane polarized light.
USP Dissolution Apparatus
• Apparatus 2 - Paddle (37º)
• Apparatus 3 - Reciprocating Cylinder (37º)
• Apparatus 4 – Flow-Through Cell (37º)
• Apparatus 5 – Paddle over Disk (32º), Transdermal Delivery
System, use paddle and vessel from Apparatus 2 with a
stainless steel disk assembly to hold the transdermal on the
bottom of vessel.
• Apparatus 6, Cylinder (32º), Transdermal Delivery System,
use Apparatus 1 except replace the basket shaft with a stainless
steel cylinder element.
• Apparatus 7, Reciprocating Holder, for transdermal delivery
systems and also a variety of dosage forms
Biopharmaceutical Classification of Drugs.
Class 2: Low Solubility - High Permeability
Class 3: High Solubility - Low Permeability
Class 4: Low Solubility - Low Permeability
Class I drugs exhibit a high absorption number and a high dissolution number. The rate limiting step is drug dissolution and if dissolution is very rapid then gastric emptying rate becomes the rate determining step.Rate of absorption is higher than rate of excretion. e.g. Metoprolol, Diltiazem, Verapamil, Propranolol.
Class II drugs have a high absorption number but a low dissolution number. In vivo drug dissolution is then a rate limiting step for absorption except at a very high dose number. The absorption for class II drugs is usually slower than class I and occurs over a longer period of time. In vitro- In vivo correlation (IVIVC) is usually excepted for class I and class II drugs. e.g. Phenytoin, Danazol, Ketoconazole, Mefenamic acid, Nifedipine.
Class III drugs, permeability is rate limiting step for drug absorption. These drugs exhibit a high variation in the rate and extent of drug absorption. Since the dissolution is rapid, the variation is attributable to alteration of physiology and membrane permeability rather than the dosage form factors. e.g. Cimetidine, Acyclovir, Neomycin B, Captopril.
Class IV drugs exhibit a lot of problems for effective oral administration. Fortunately, extreme examples of class IV compounds are the exception rather than the rule and are rarely developed and reach the market. Nevertheless a number of class IV drugs do exist. e.g. Taxol, Griseofulvin.
Note:
- Absorption no is ratio of mean residence time to mean absorption time.
- Dissolution no is ratio of mean residence time to mean dissolution time.
Selection of Branch for Masters
- Pre-clinincal and Clinical research in Clinical Research Organization(CRO)
- Bioavaibility and Bioequivelence study(BA-BE)
- Pharmacological Screening in Drug development
- Animal modeling in Drug discovery
- Bio assay
- Formulation and Development
- Regulatory affairs
- Intellectual Property Rights
- Production of formulation
- Quality Assurance
- Solid state characterization
- Pharmacokinetic
- Basic Research in pharmaceutics
- Drug Discovery and Development
- Lead Optimization
- Pro-drug modification
- Intellectual Property Rights
- Structure Elucidation
- Characterization and evaluation of plants,its parts and product
- Extraction,characterization and evaluation of active constituents from plant.
- Structure Elucidation of active constituents in plant.
- Formulation of Herbs and Extract
- TLC,HPLC,HPTLC,Mass,NMR etc
- Qualitative and Quantitative analysis of API
- Impurity profiling in API
- Metabolite characterization
- Bioanalysis from blood and body fluids.
- Analysis Method development
- Vector construction
- Delivery of vector and genes
- Measurement of Expression of genes
- Bioassay in HTS
- Identification of Gene in population
- Different types of PCR
- Protein Purification
- Fermentation
Gene Therapy
Types of gene therapy
1. Germ line gene therapy :
Germ cells, i.e., sperm or eggs, are modified by the introduction of functional genes, which are integrated into their genomes. Therefore, the change due to therapy would be heritable and would be passed on to later generations.
2.Somatic gene therapy:
The therapeutic genes are transferred into the somatic cells of a patient. Any modifications and effects will be restricted to the individual patient only, and will not be inherited by the patient's offspring or later generations.
Vectors in gene therapy
1. Viruses
Retroviruses
Adenoviruses
Envelope protein pseudotyping of viral vectors
Replication-Competent Vectors
Cis and trans-acting elements
Herpes Simplex Virus
Injection of Naked DNA
4.Chemical Methods to Enhance Delivery
4.Chemical Methods to Enhance Delivery
Magnetofection
Oligonucleotides
Lipoplexes and polyplexes
Dendrimers
Bredt's rule
For example, two of the following isomers of norbornene violate Bredt's rule, which makes them too unstable to prepare:
In the figure, the bridgehead atoms involved in Bredt's rule violation are highlighted in red.
Bredt's rule is a consequence of the fact that having a double bond on a bridgehead would be equivalent to having a trans double bond on a ring, which is not possible for small rings (fewer than eight atoms) due to ring strain, and angle strain in particular.
Bredt's rule can be useful for predicting which isomer is obtained from an elimination reaction in a bridged ring system. It can also be applied to reaction mechanisms that go via carbocations and, to a lesser degree, via free radicals, because these intermediates, like carbon atoms involved in a double bond, prefer to have a planar geometry with 120 degree angles and sp2 hybridization.
An anti-bredt molecule is one that is found to exist and be stable (within certain parameters) despite this rule. A recent (2006) example such a molecule is 2-quinuclidonium tetrafluoroborate.
MCQ : Important for NIPER JEE
1. Which of the following compounds would have the highest boiling point?
(a) CH3CH2CH2CH3
(b) CH3NH2
(c) CH3OH
(d) CH2F2
Answer:C
Explaination:This compound (methanol) forms the strongest hydrogen bonds of the available choices since oxygen is more electronegative than nitrogen. Although fluorine is more electronegative than oxygen there are no HF bonds in answer (d). Methanol also has some of the strongest dipole-dipole interactions of the available compounds although hydrogen bonding is the principle intermolecular attraction when a compound has an electropositive hydrogen atom and available non-bonding electron pairs.
2. The most stable conformational isomer of cis-1-bromo-2-chlorocyclohexane will have...
(a) both halide atoms in axial positions.
(b) both halide atoms in equatorial positions.
(c) the bromine atom in an axial position and the chlorine atom in an equatorial position.
(d) the bromine atom in an equatorial position and the chlorine atom in an axial position.
Answer:D
Explaination:The bromine atom is larger than the chlorine atom and should be placed in an equatorial position. The cis geometry requires that the chlorine atom be placed in an axial position.
3. How many dichlorinated isomers can be formed by the halogenation of CH3CH2CH2CH3 with Cl2 in the presence of light?
(a) 2
(b) 3
(c) 5
(d) 6
Answer:D
Explaination:The correct isomers are...1,1...1,2...1,3...1,4...2,2...2,3 dichlorobutanes
4. The CMR spectrum of an unknown compound shows 6 absorptions and the PMR spectrum shows 5 absorptions. Which of the following compounds is the unknown compound?
Answer:B
5.The most stable conformational isomer of trans-1-ethyl-2-methylcyclohexane will be...
Answer:C
6. The best nomenclature for the geometry of the following compound is...
(a) 1,3-pentamethylpropane
(b) 1,1,3,3-tetramethylbutane
(c) 2,4,4-trimethylpentane
(d) 2,2,4-trimethylpentane
Answer:C
Explaination:The priority of the 1-methylethyl group is higher than the 1-propyl group thus making the compound an E for "entgegen" (opposite in German).
7. Arrange the following groups in decreasing order of priority for E/Z nomenclature with the highest priority group listed first.
(a) B>A>C
(b) B>C>A
(c) A>C>B
(d) C>A>B
Answer:A
Explaination:The fluorine atom has the highest atomic mass and must be first followed by the isopropyl group, which by being branched has a higher priority than the pentyl group.
8.Which of the following is an intermediate in the reaction of benzene with CH3Cl and AlCl3?
Answer:C
Explaination:Aluminum chloride is a strong Lewis acid and reacts with methyl chloride to form a methyl carbocation, which is the electrophile in the next step of the reaction.
9. Which of the following represents the best resonance form for H2C=CHF?
Answer:C
Explaination:The fluorine atom must donate an electron pair to the double bond.
10.Which of the following compounds is the strongest Brønsted base?
(a) CH4
(b) NH3
(c) H2O
(d) HF
Answer:B
Explaination:Ammonia is the strongest base in this group. It has a non-bonded pair of electrons and has the lowest electronegativity in the group of compounds that has non-bonded electron pairs.
11. Which of the following compounds is the strongest Brønsted base?
(a) H2PO4-
(b) HSO4-
(c) NO3-
(d) CH3COO-
Answer: D
Explaination:All of the other bases have a full positive charge on the atom adjacent to the oxygen atom that has the negative charge. This will attract the electrons on the charged oxygen atom and make them less available for sharing. Another way to remember this is that the acetate ion will be the strongest base because acetic acid is the weakest acid in the group. A strong acid must logically create a weak conjugate base or the acid could not give up the proton readily.
Blot test
It uses gel electrophoresis to separate native or denatured proteins by the length of the polypeptide (denaturing conditions) or by the 3-D structure of the protein (native/ non-denaturing conditions). The proteins are then transferred to a membrane (typically nitrocellulose or PVDF), where they are probed (detected) using antibodies specific to the target protein.
Southern blot test:To detect DNA in sample.
Northern blot test:To detect RNA in sample.
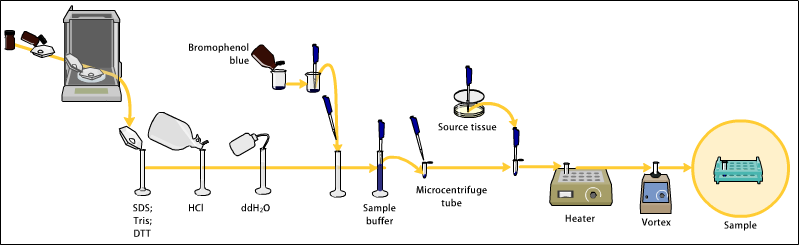
General Procedure
Gel electrophoresis
Gel electrophoresis refers to using a gel as an anticonvective medium and or sieving medium during electrophoresis.
Gel electrophresis is most commonly used for separation of biological macromolecules such as deoxyribonucleic acid (DNA), ribonucleic acid (RNA), or protein; however, gel electrophoresis can be used for separation of nanoparticles.
Electrophoresis refers to the movement of a charged particle in an electrical field.
Gels suppress the thermal convection caused by application of the electric field, and can also act as a sieving medium, retarding the passage of molecules; gels can also simply serve to maintain the finished separation, so that a post electrophoresis stain can be applied.
DNA Gel electrophoresis is usually performed for analytical purposes, often after amplification of DNA via PCR, but may be used as a preparative technique prior to use of other methods such as mass spectrometry, RFLP, PCR, cloning, DNA sequencing, or Southern blotting for further characterization.
Interpretation